What Are We Doing?
It is little wonder that some kids don't want to do geography. We make it so impossible at times. I have my views as to why, so read on to find out more. Apologies in advance for throwing a few rocks!

Grey lathes of plagioclase feldspar, with smaller pyroxene and olivine crystals within the groundmass. This rock is a basalt.
Hacking through the text books on geography really does make my heart sink. I guess I am in privileged position, being on the other side of the hump so to speak. Why do I say this? Well, my usual hobby-horse of plate tectonics springs to mind. It has been demoted, relegated, reduced to sitting there along with fluid dynamics and weathering – like an equal. This is a nonsense, for without plate tectonics we would not have a home – there would be no land, there would be no mountains, there would be no atmosphere, and perhaps there would be no 'us'. Whether the latter is a good or bad thing is a topic I am not going to debate here. It is the exquisitely beautiful, fine-tuned operating system that built our planet, keeps it dynamic and recycles everything. Without it we would have no fluvial systems, weather or human geography, so this topic has to be elevated to be the overarching theory – once this is understood, everything falls into place.
The whole lot gets brought to boil and magic takes place in that pot
Another topic which dismayed me is that of weathering. Imagine a confectioner making fudge. Into the pot does sugar, water, chocolate, cream and maybe some liqueur. The whole lot gets brought to boil and magic takes place in that pot. Then it gets poured out to cool. Imagine now if we could cool it really slowly and give the crystals time to grow. I think you will agree with me that sugar has a different melting point to chocolate which has a different melting point to water, and alcohol has the lowest melting point of all and will have evaporated off at the start of the process.

An augite crystal
Taking specific elements out of the mix and denying them to other minerals
But the point is this – different crystals will form from the various constituents as the temperature falls and they pass through their ‘freezing’ points. A similar thing happens when you thaw a frozen bottle of milk – the water thaws first and the solids come later. Igneous rocks are the same – certain minerals solidify at certain temperatures, and those stable at the higher temperatures form first, and so on as the magma or lava cools. Bear in mind that the chemistry of the melt – which is the term that igneous petrologists use for the cooling magma – changes as minerals form, taking specific elements out of the mix and denying them to other minerals further down the cooling curve. Eventually only silica is left.

A quartz crystal
It is little wonder that kids don’t want to do geography.
So, what has this got to do with weathering? Well, everything. I saw a diagram of Goldich’s weathering system in the text books. This is okay, but what does it mean? There is a list of minerals – olivine – augite-hornblende-biotite – orthoclase-quartz on the right-hand side, and lime plagioclase - sodic plagioclase on the left of the diagram. My heart sinks when I see this, and it is little wonder that kids don’t want to do geography. Whew! What is going on here? A very famous igneous petrologist called Norman Bowen worked out the various minerals that formed as a magma cooled – which is essentially Goldich’s system. Bowen’s Reaction Series is one of the first things that an undergraduate geology student learns. So, Bowen’s Reaction Series was the groundwork that established the direct line of descent of minerals forming in a melt. Weathering works in reverse.

Norman Bowen - what a hero!
This is partly true, but worse, it does nothing to convey the magic
What worries me are statement like this – “many minerals are formed under high pressure and high temperature in the Earth’s core. As they cool, they become more stable.” This is partly true, but worse, it does nothing to convey the magic that goes into the formation of minerals. Minerals that crystallise in the early stages of rock formation do so at higher temperatures. For the record, the pressures will probably be the same during the process unless there is an eruption. As temperatures fall, new minerals form as they pass through their freezing points, as we have seen earlier.
Those minerals formed at higher temperatures are the least stable
When these same rocks are exposed at the surface and subject to the agents of erosion – water, heat, wind, mechanical break up to name an obvious few, it is those minerals that formed at higher temperatures that are the least stable, because the ambient temperatures in which the rock finds itself is very different from the temperature of their formation.

A petrological microscope
The formula for augite is (Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6
They are also more susceptible to weathering thanks to their inherent softness and complex chemistry. The formula for augite – a pyroxene mineral – is (Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6 whilst that of the most stable common mineral, quartz, is SiO2.
They are just a load of horrible names to learn
And what on Earth is olivine, augite, hornblende, biotite, orthoclase, plagioclase, muscovite and quartz anyway? I know, having peered down petrological microscopes for 4 years, but for our dear geography scholars it means nothing – just a load of horrible names to learn to regurgitate in the examinations. Grrrrrrrrrrrrrrr!
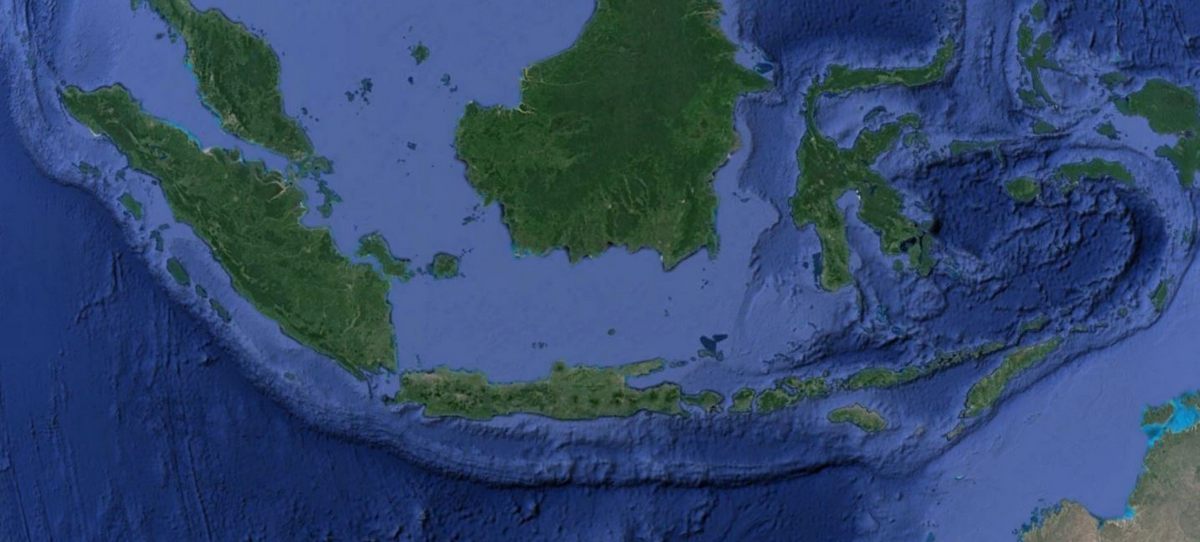
An Island Arc - the Islands of Sumatra, Java, Bali, Lombok Komodo, Nusa Tengarra and Flores.
Let’s take a look at Bowen’s experimental setup
I suggest we take a look at some of those minerals, both as crystals that can be seen with the naked eye, and in thin section under a petrological microscope. Let’s take a look at Bowen’s experimental setup in the Geophysical Laboratory at the Carnegie Institution to see what he got up to there, and let us go and have some fun around the island arcs of Indonesia and Japan when it comes to studying plate tectonics.

